PRODUCT >> Intermediates
|
Product Name |
CAS No. |
|
5,16-Androstadien-3beta-ol |
1224-94-8 |
Product Name (品名): | 5,16-Androstadien-3beta-ol 雄甾-5,16-二烯-3β-醇 |
Molecular Formula (分子式): | C19H28O |
Molecular Weight (分子量): | 272.43 |
Purity (纯度): | 98% |
| Production Capacity (产能) | 500KG/ Year |
Specification:(指标) | MS, HNMR, Purity 99% by HPLC |
Contact Telephone (联系电话): | 023-677770219 |
Contact | Sales Department |
Contact Email: | sales@chemieliva.com |
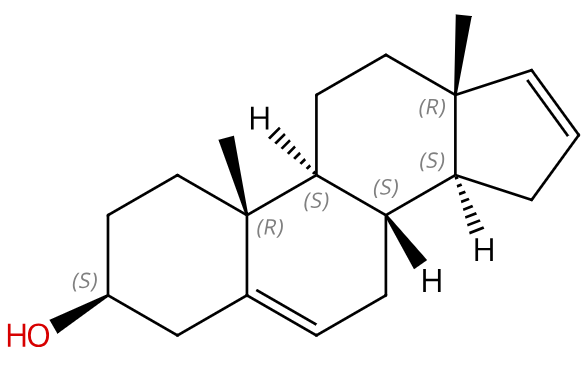
Structure (结构式):
| |
Description | 5,16-Androstadien-3beta-ol (5,16-AD3) is a naturally occurring steroid hormone that is produced in the human body. It has recently been studied for its potential therapeutic applications due to its ability to target specific receptors in the body and its ability to interact with other hormones. 5,16-AD3 has been found to have a variety of effects on the body, including the regulation of metabolic processes, the modulation of immune system responses, and the control of inflammation. This paper will discuss the synthesis method, scientific research application, mechanism of action, biochemical and physiological effects, advantages and limitations for lab experiments, and future directions for 5,16-AD3 research. |
Synthesis Method | 5,16-Androstadien-3beta-ol can be synthesized from a variety of starting materials. It can be synthesized from cholesterol, progesterone, and pregnenolone, as well as from androstenedione, androstenediol, and androstadienol. The synthesis of 5,16-Androstadien-3beta-ol involves a series of chemical reactions, including hydrogenation, oxidation, and cyclization. The synthesis of 5,16-Androstadien-3beta-ol can be done in a laboratory setting using a variety of techniques, including gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC). |
Synthesis Method Details | Design of the Synthesis Pathway The synthesis pathway of 5,16-Androstadien-3beta-ol involves the conversion of pregnenolone to androstadienone, followed by the reduction of androstadienone to 5,16-Androstadien-3beta-ol. Starting Materials Pregnenolone, Sodium borohydride, Acetic acid, Methanol, Hydrochloric acid, Sodium hydroxide Reaction Pregnenolone is converted to androstadienone by treatment with acetic acid and methanol., Androstadienone is reduced to 5,16-Androstadien-3beta-ol using sodium borohydride as a reducing agent., The reaction is carried out in methanol with the addition of hydrochloric acid as a catalyst., The reaction mixture is then neutralized with sodium hydroxide and the product is extracted with a suitable solvent. |
Scientific Research Application | 5,16-Androstadien-3beta-ol has been studied for its potential therapeutic applications in a variety of conditions, including obesity, diabetes, and cardiovascular disease. It has also been studied for its potential role in the regulation of metabolic processes, the modulation of immune system responses, and the control of inflammation. In addition, 5,16-Androstadien-3beta-ol has been studied for its potential use in the treatment of neurological disorders, such as Alzheimer's and Parkinson's disease. |
Mechanism of Action | 5,16-Androstadien-3beta-ol binds to a variety of receptors in the body, including the androgen receptor, the glucocorticoid receptor, and the progesterone receptor. 5,16-Androstadien-3beta-ol has been found to interact with other hormones, such as testosterone, progesterone, and cortisol, to regulate the body's metabolic processes, immune system responses, and inflammation. |
Biochemical and Physiological Effects | 5,16-Androstadien-3beta-ol has been found to have a variety of biochemical and physiological effects on the body. It has been found to have anti-inflammatory, anti-diabetic, and anti-obesity effects. It has also been found to have a positive effect on the cardiovascular system, as well as on the immune system and the nervous system. |
Advantages and Limitations for Lab Experiments | The advantages of using 5,16-Androstadien-3beta-ol in laboratory experiments include its ability to target specific receptors in the body, its ability to interact with other hormones, and its ability to regulate metabolic processes, immune system responses, and inflammation. The limitations of using 5,16-Androstadien-3beta-ol in laboratory experiments include its short half-life, its potential for toxicity, and its potential for side effects. |
Future Directions | Future directions for 5,16-Androstadien-3beta-ol research include further exploration of its potential therapeutic applications in a variety of conditions, including obesity, diabetes, and cardiovascular disease. In addition, further research is needed to better understand the mechanisms of action of 5,16-Androstadien-3beta-ol, as well as its biochemical and physiological effects. Additionally, further research is needed to explore the potential advantages and limitations of using 5,16-Androstadien-3beta-ol in laboratory experiments. Finally, further research is needed to explore the potential of 5,16-Androstadien-3beta-ol as a therapeutic agent in the treatment of neurological disorders, such as Alzheimer's and Parkinson's disease. |
CAS RN | 1224-94-8 |
Product Name | 5,16-Androstadien-3beta-ol |
Molecular Formula | C19H28O |
Molecular Weight | 272.4 g/mol |
IUPAC Name | (3S,8S,9S,10R,13R,14S)-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15-decahydro-1H-cyclopenta[a]phenanthren-3-ol |
InChI | InChI=1S/C19H28O/c1-18-9-3-4-16(18)15-6-5-13-12-14(20)7-11-19(13,2)17(15)8-10-18/h3,5,9,14-17,20H,4,6-8,10-12H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 |
InChI Key | QVHPTAJAHUONLV-DYKIIFRCSA-N |
Isomeric SMILES | C[C@]12CC[C@H]3[C@H]([C@@H]1CC=C2)CC=C4[C@@]3(CC[C@@H](C4)O)C |
SMILES | CC12CCC3C(C1CC=C2)CC=C4C3(CCC(C4)O)C |
Canonical SMILES | CC12CCC3C(C1CC=C2)CC=C4C3(CCC(C4)O)C |
Synonyms | 5,16-androstadien-3 beta-ol andien-beta androsta-5,16-dien-3 beta-ol androstadienol |
Origin of Product | China |

重庆福腾医药化工有限公司